Theory and Defination :
The reduction of aromatic substrates with alkali metals, alcohol in liquid ammonia is known as "Birch reduction". This reaction is named after a Australian chemist Arthur John Birch in 1944. Aromatic rings can be completely reduced by catalytic hydrogenation. But, when aromatic rings are reduced by sodium or lithium in liquid ammonia and in the presence of ethanol or methanol, the aromatic ring is only partially reduced. This reaction is one of the most fundamental reactions in organic chemistry and called Birch reduction.
General Reaction with illustration :
The reaction is usually conducted at the boiling point of ammonia. A familiar example involves the conversion of benzene to 1, 4-dihydrobenzene in the presence of Na or Li/NH3.
Mechanism:
The question of why the 1,3-diene is not formed, even though it would be more stable through conjugation, can be rationalized with a simple mnemonic. When viewed in valence bond terms, electron-electron repulsions in the radical anion will preferentially have the nonbonding electrons separated as much as possible, in a 1,4-relationship.
This question can also be answered by considering the mesomeric structures of the dienyl carbanion:

The numbers, which stand for the number of bonds, can be averaged and compared with the 1,3- and the 1,4-diene. The structure on the left is the average of all mesomers depicted above followed by 1,3 and 1,4-diene:

The difference between the dienyl carbanion and 1,3-diene in absolute numbers is 2, and between the dienyl carbanion and 1,4-diene is 4/3. The comparison with the least change in electron distribution will be preferred.
Example and Application :
1) Reactions of arenes with +I- and +M-substituents lead to the products with the most highly substituted double bonds:

2) The effect of electron-withdrawing substituents on the Birch Reduction varies. For example, the reaction of benzoic acid leads to 2,5-cyclohexadienecarboxylic acid, which can be rationalized on the basis of the carboxylic acid stabilizing an adjacent anion:

3) Alkene double bonds are only reduced if they are conjugated with the arene, and occasionally isolated terminal alkenes will be reduced.


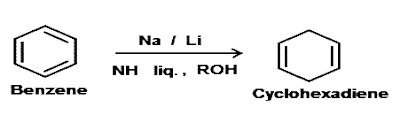
0 Comments:
Post a Comment